Debut of China’s Innovative Medical Device in India | Prof. Wang Yan’s Team from XMCH Demonstrates Challenging Transcatheter Tricuspid Valve Repair Using the NeoBlazar® System
18 June,2025
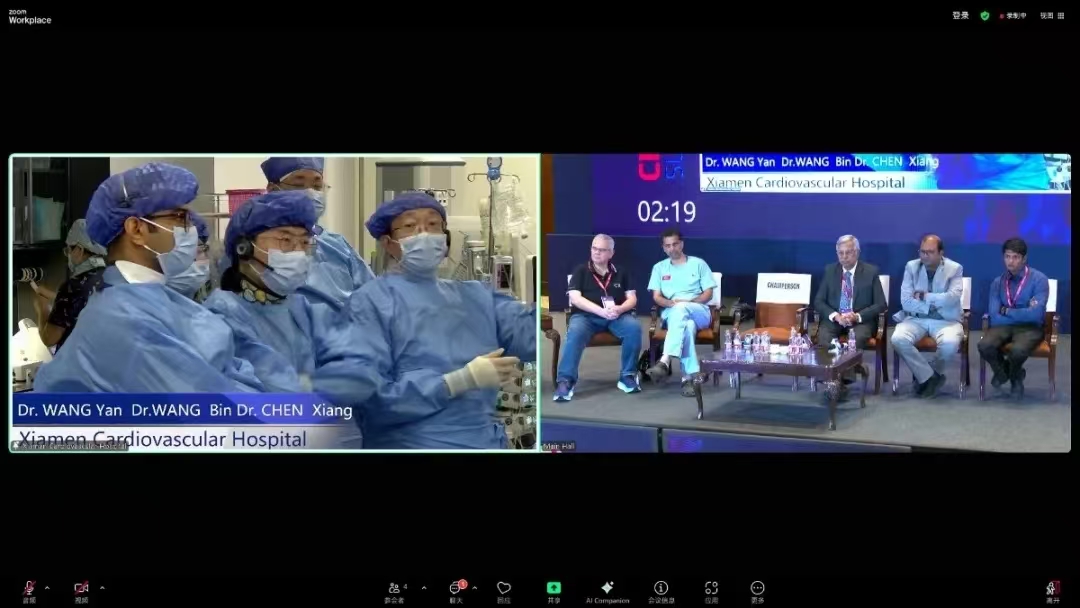
From June 12 to 14, the 6th Complex High-Risk Interventional Procedures (CHIP) INDIA SUMMIT 2025 was held in Chennai, India. Invited by the organizers, Professor Wang Yan’s team from Xiamen Cardiovascular Hospital Xiamen University (XMCH), performed a live broadcast of a complex transcatheter edge-to-edge tricuspid valve repair (T-TEER) using the domestically developed NeoBlazar® system. Prof. Wang also delivered a keynote speech titled “Emerging Therapies in Structural Intervention - 2025 Update”, showcasing the outstanding performance of Chinese innovative devices and sharing China’s solutions and expert experience in the field of structural heart interventions.

The CHIP INDIA SUMMIT is one of the most influential conferences in the field of complex high-risk coronary interventions in India and South Asia. It brings together top cardiovascular experts from India and around the world to share innovative interventional techniques and breakthroughs through academic presentations, case demonstrations, expert panel discussions, and hands-on practice. Currently, tricuspid valve interventions are still in the early stages in India. As an invited International Course Director, Professor Wang Yan specifically demonstrated the Chinese innovative tricuspid device, sharing cutting-edge progress and clinical experience from XMCH, which was highly praised by attending international experts. Notably, three Indian scholars participating in Heart Sampling Visiting Scholar Program at XMCH were also present for the live surgery broadcast.
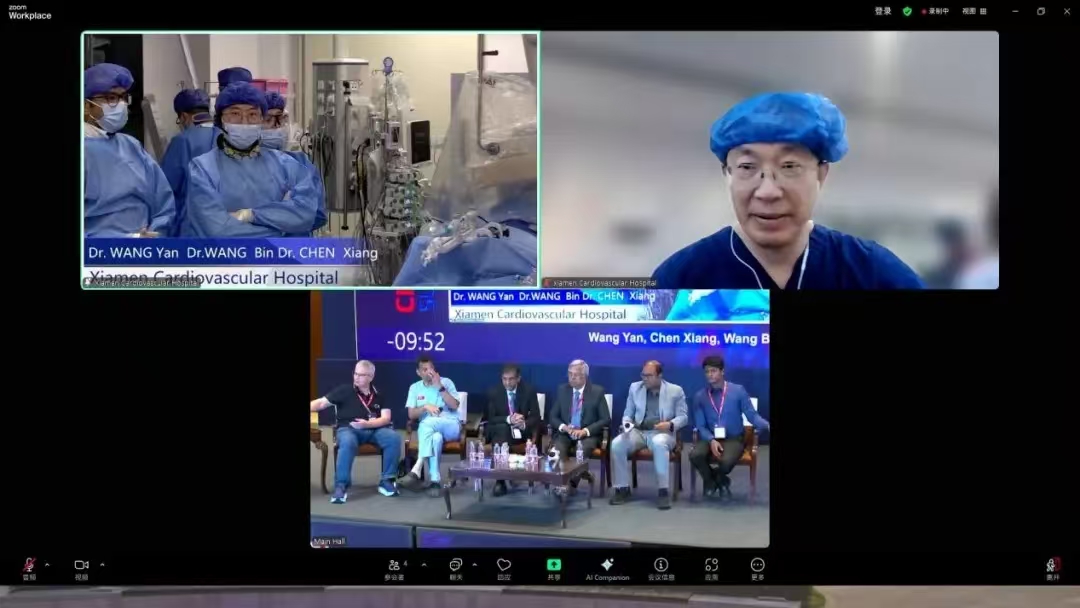
As China’s first T-TEER system, NeoBlazar® was co-developed by Professor Wang Yan’s team in collaboration with a domestic innovative medical device company, and nationwide multicenter clinical trials were conducted under his team’s leadership. Of particular note, the demonstrated procedure was part of an investigator-initiated clinical trial (IIT), aimed at identifying and improving critical clinical issues related to diagnosis, treatment, and prevention. IITs address more diverse clinical questions than standard clinical trials and play a significant role in advancing both academic research and industrial development. Currently, NeoBlazar® has successfully passed the NMPA’s Special Review for Innovative Medical Devices and is expected to bridge the gap in the area of interventional therapy in china, offering a new treatment option for patients with tricuspid regurgitation.
share
Latest News
-
01 December,2025
Heart Saplings Blossom| Fellows from India and Indonesia Win Awards at International Academic Conferences with Cases from XMCH
-
03 November,2025
“Made-in-China” Robot Performs Cross-Continental Heart Intervention, Achieving a World First in Remote Operations
-
09 October,2025
Accelerating Technology Export | Xiamen experts conduct academic exchanges and demonstrate domestic IVL procedure in multiple hospitals in Pakistan

